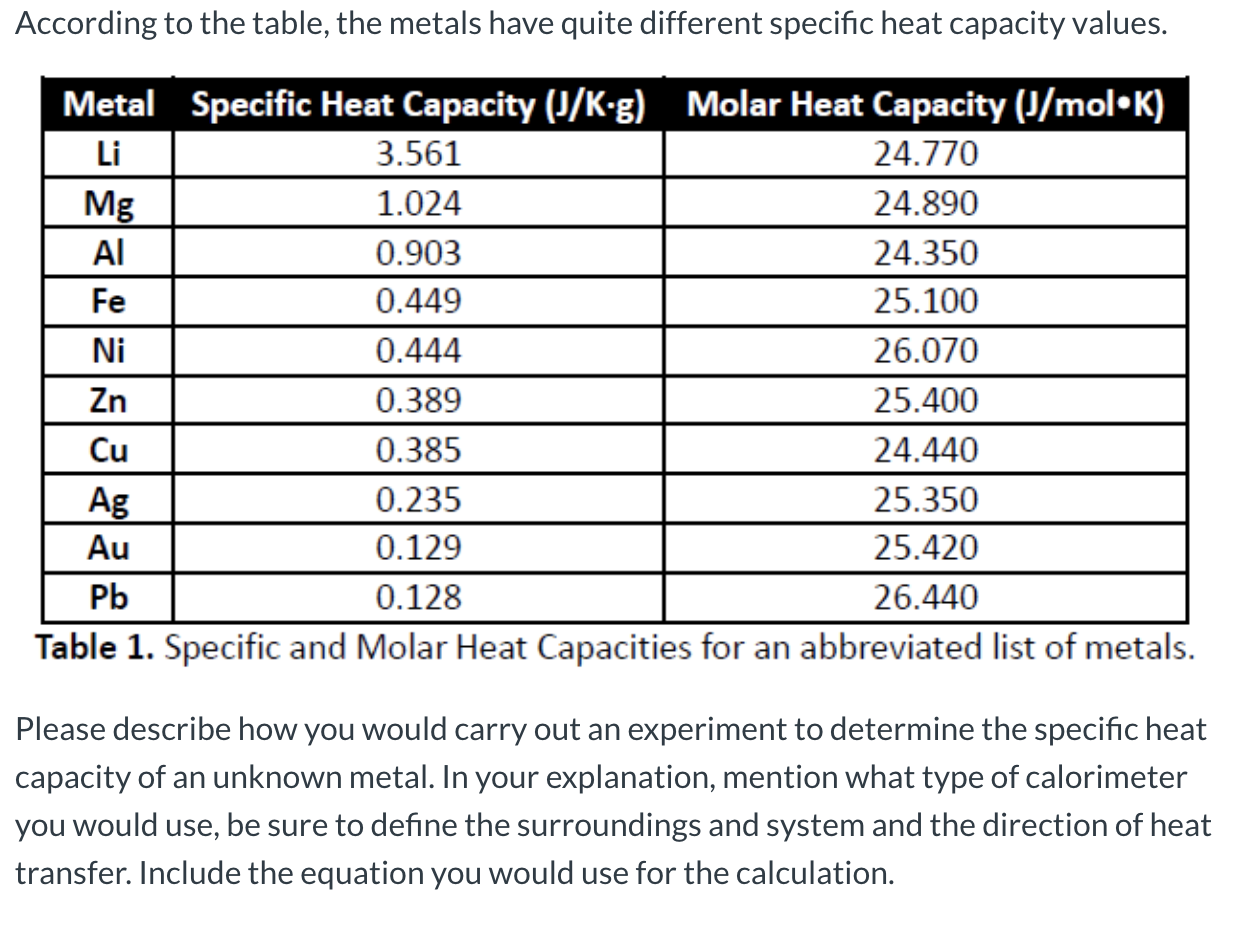
![PDF] A NEW CORRELATION FOR THE SPECIFIC HEAT OF METALS, METAL OXIDES AND METAL FLUORIDES AS A FUNCTION OF TEMPERATURE | Semantic Scholar PDF] A NEW CORRELATION FOR THE SPECIFIC HEAT OF METALS, METAL OXIDES AND METAL FLUORIDES AS A FUNCTION OF TEMPERATURE | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b0d76592f078aeeb6e66f500b45a1f101c6fe150/5-Table1-1.png)
PDF] A NEW CORRELATION FOR THE SPECIFIC HEAT OF METALS, METAL OXIDES AND METAL FLUORIDES AS A FUNCTION OF TEMPERATURE | Semantic Scholar
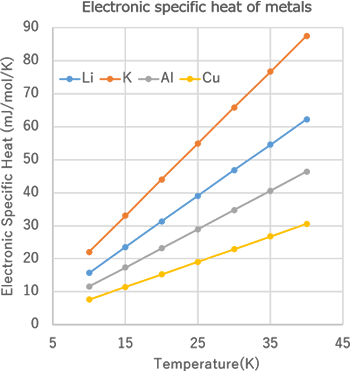
Electronic specific heat analysis of metals - J-OCTA Case Studies | CAE Solutions - JSOL Corporation

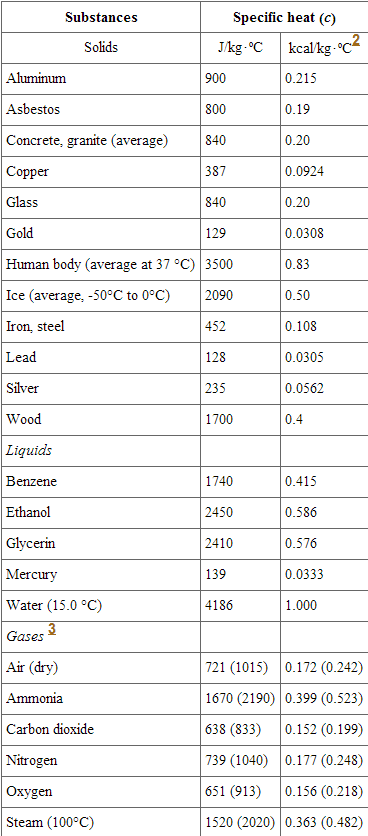
and specific heats of various metals; temperature increments in those... | Download Scientific Diagram

PDF) A new correlation for the specific heat of metals, metal oxides and metal fluorides as a function of temperature
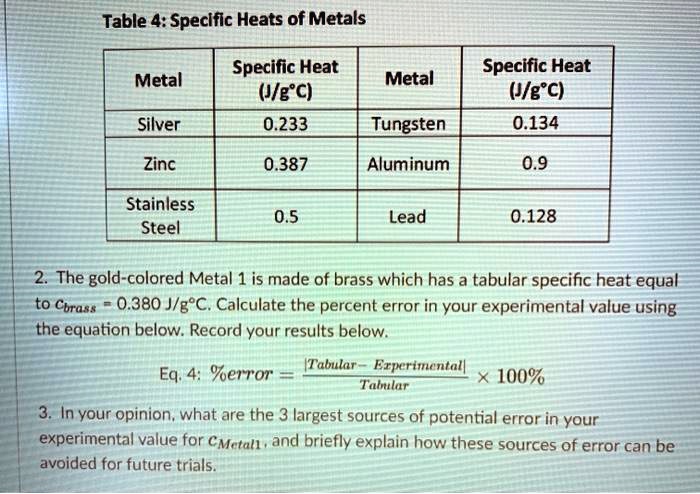
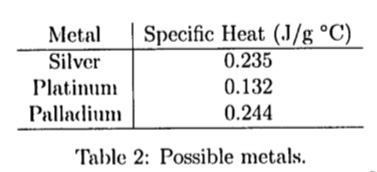
SOLVED: Table 4: Specific Heats of Metals Metal Specific Heat (J/g°C) Tungsten 0.233 Silver 0.134 Zinc 0.387 Aluminum 0.9 Stainless Steel 0.5 Lead 0.128
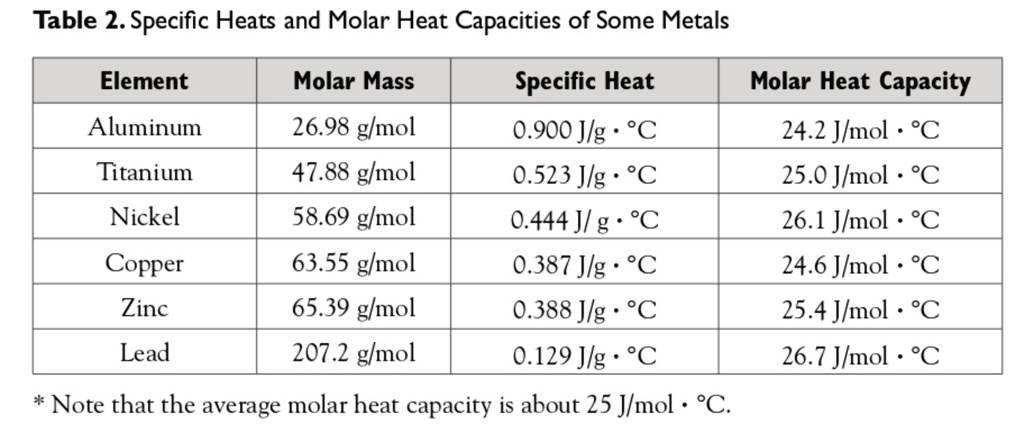
SOLVED: Table 2. Specific Heats and Molar Heat Capacities of Some Metals Element Molar Mass Specific Heat Molar Heat Capacity Aluminum 26.98 g/mol 0.900 J/g°C 24.2 J/mol°C Titanium 47.88 g/mol 0.523 J/g°C
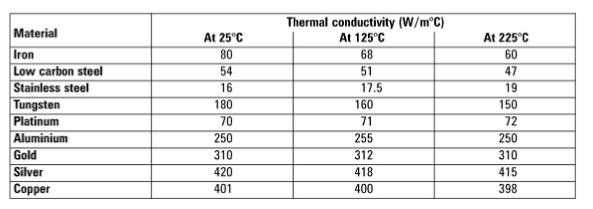
Which metal achieves the highest temperature when adding energy? Aluminum, Copper, or Silver? | CIDER
![PDF] A NEW CORRELATION FOR THE SPECIFIC HEAT OF METALS, METAL OXIDES AND METAL FLUORIDES AS A FUNCTION OF TEMPERATURE | Semantic Scholar PDF] A NEW CORRELATION FOR THE SPECIFIC HEAT OF METALS, METAL OXIDES AND METAL FLUORIDES AS A FUNCTION OF TEMPERATURE | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b0d76592f078aeeb6e66f500b45a1f101c6fe150/7-Table4-1.png)
PDF] A NEW CORRELATION FOR THE SPECIFIC HEAT OF METALS, METAL OXIDES AND METAL FLUORIDES AS A FUNCTION OF TEMPERATURE | Semantic Scholar