
Auditing Organization (AO) versus Notified Body (NB) versus Registrar. What's the difference? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog

CE Mark for All Directives by EU Notified Bodies at Rs 20000/certificate in Palghar | ID: 4438471733

Role of Notified Bodies in Assessing Clinical Evaluation Reports (CERs) under EU MDR — OMC Medical Limited | by Omcmedical Marketing | Mar, 2024 | Medium
![Requirements Relating to Notified Bodies for EU MDR [Video] - LearnGxP: Accredited Online Life Science Training Courses Requirements Relating to Notified Bodies for EU MDR [Video] - LearnGxP: Accredited Online Life Science Training Courses](https://learngxp.com/wp-content/uploads/2021/04/ELM-320-01-Requirements-Relating-to-Notified-Bodies-for-EU-MDR.png)
Requirements Relating to Notified Bodies for EU MDR [Video] - LearnGxP: Accredited Online Life Science Training Courses
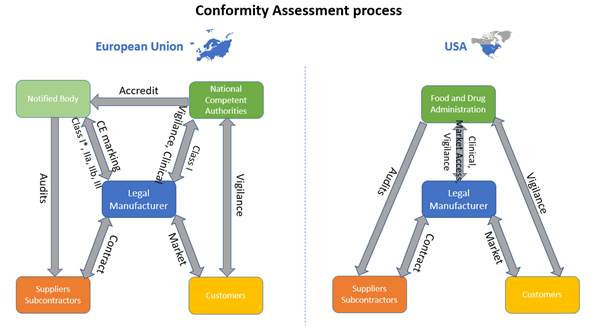
What are the principal differences between the conformity assessment process of a medical device in the USA and in the European Union? - Kvalito





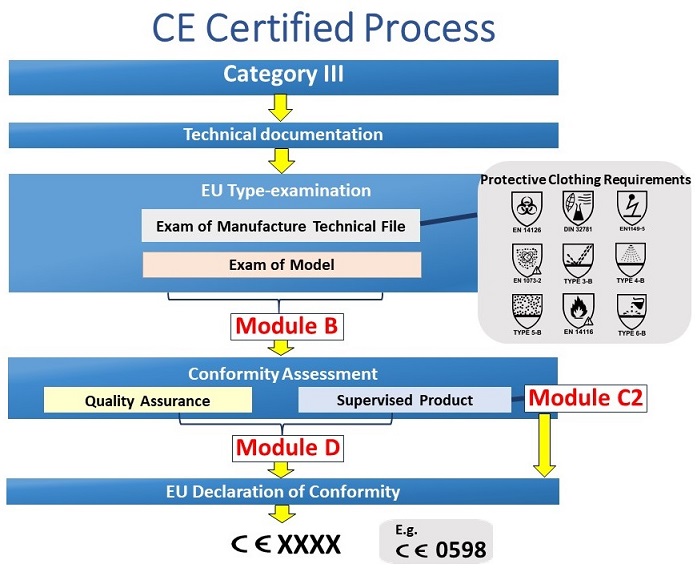







.jpg)




