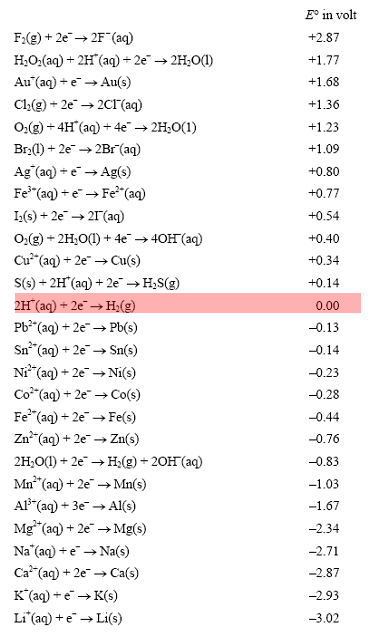Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25°C | Semantic Scholar

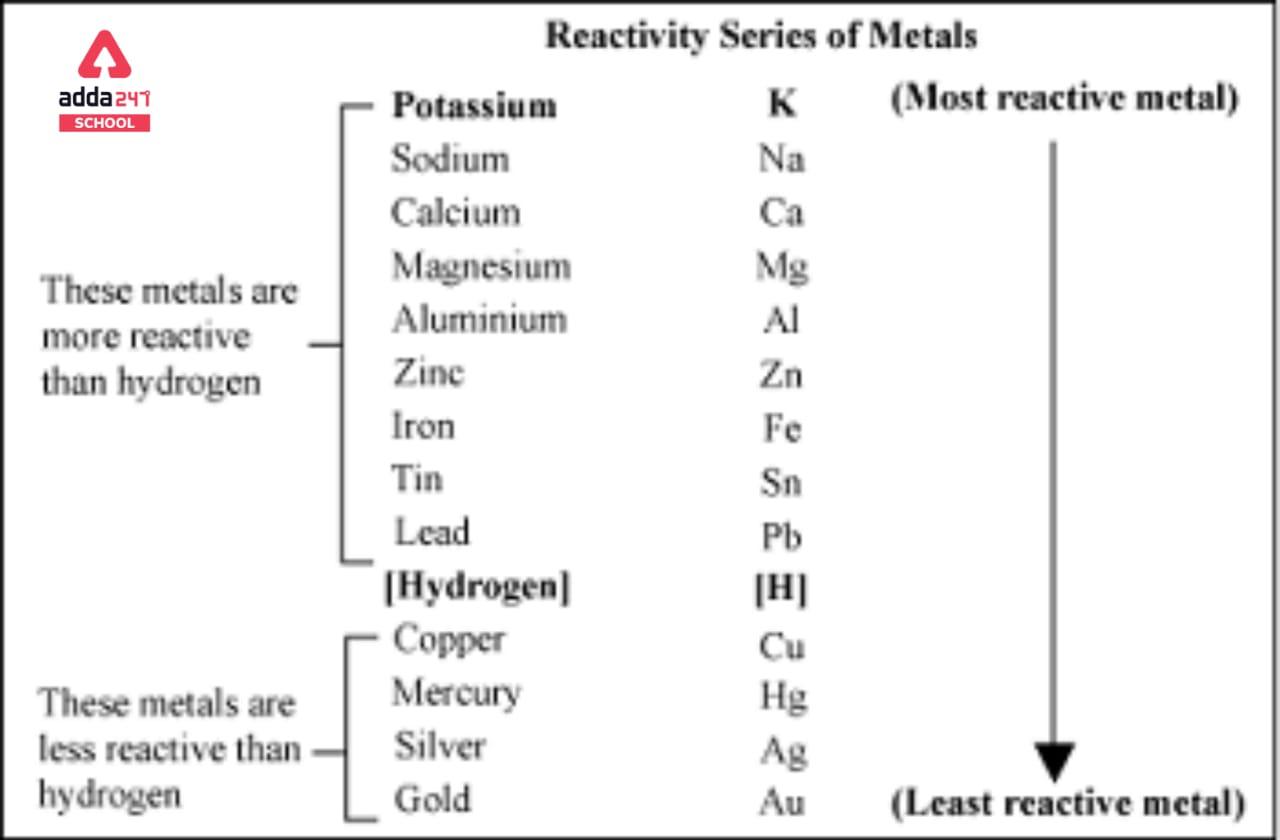
Explain the difference between the electrochemical series and metal activity series Explain why sodium is placed below calcium in electrochemical - Chemistry - Electrolysis - 13786859 | Meritnation.com
![PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/56964684a624c5af38c7e62256db3faa4c542d88/19-Table2-1.png)
PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar

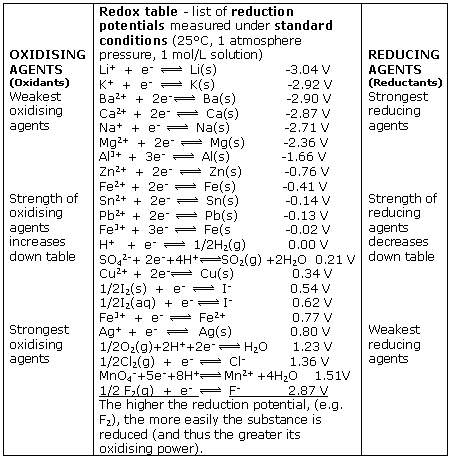
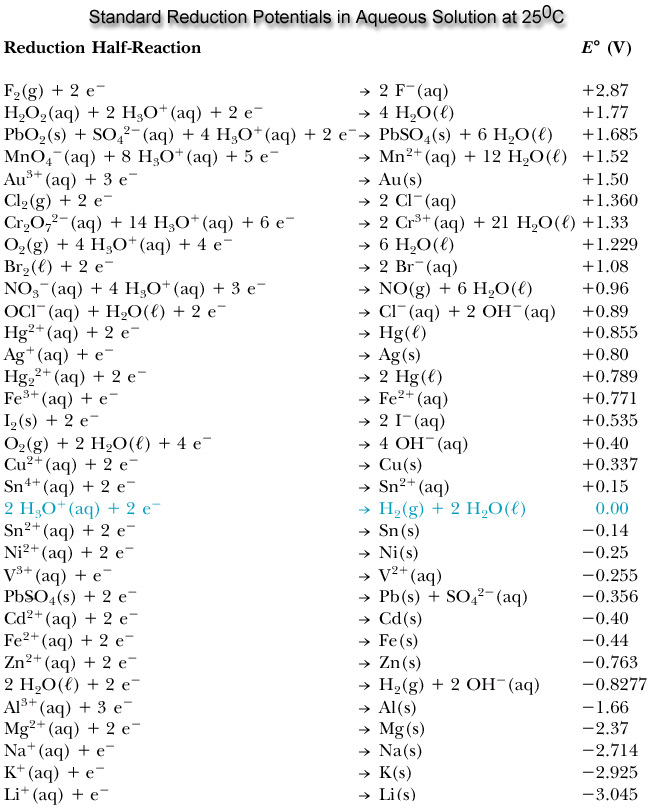
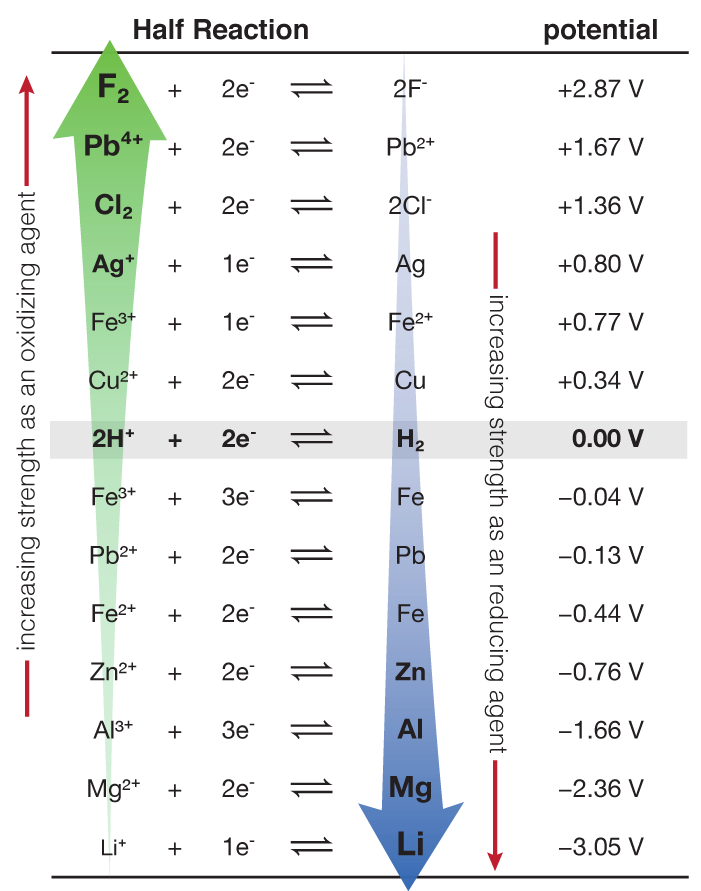
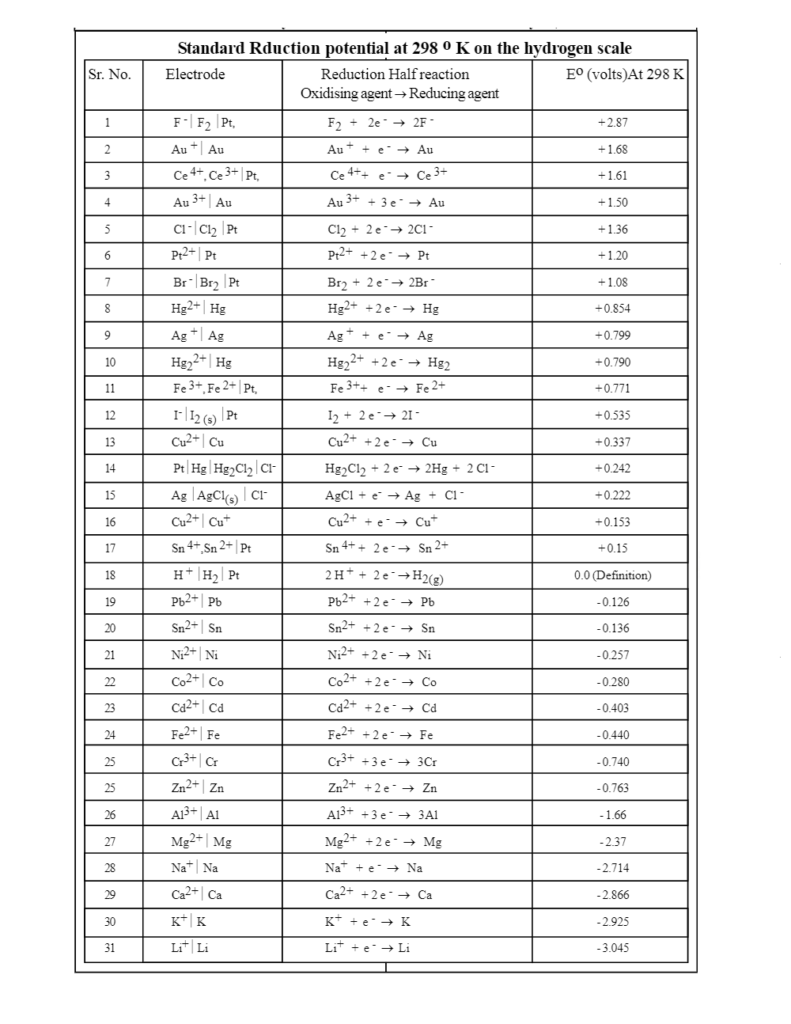
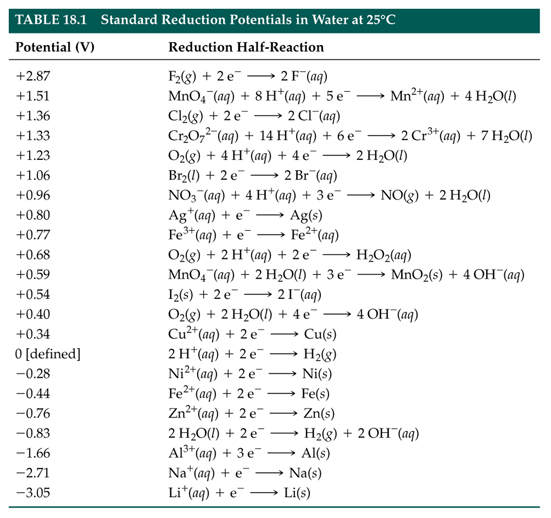
Using the standard electrode potentials given in Table 3.1, predict the reaction between the following is feasible:(i) Fe^{3+}(aq) and I^{-}(aq)(ii) Ag^{+} (aq) and Cu(s)(iii) Fe^{3+} (aq) and Br^{-} (aq)(iv) Ag(s) and Fe^{3+} (